Meracine factory has sophisticated technology, production lines, and a system of records and papers that meet World Health Organization (WHO) GMP, GLP, and GSP requirements. We always strictly control the production process of each product from raw materials, research, production, and testing to the finished product with a team of professional staff and specialists. Meracine believes that this is the right path to achieving a sustainable and reputable development strategy in line with the company’s “dedicated to health” corporate philosophy.

Meracine factory has sophisticated technology, production lines, and a system of records and papers that meet World Health Organization (WHO) GMP, GLP, and GSP requirements. We always strictly control the production process of each product from raw materials, research, production, and testing to the finished product with a team of professional staff and specialists. Meracine believes that this is the right path to achieving a sustainable and reputable development strategy in line with the company’s “dedicated to health” corporate philosophy.

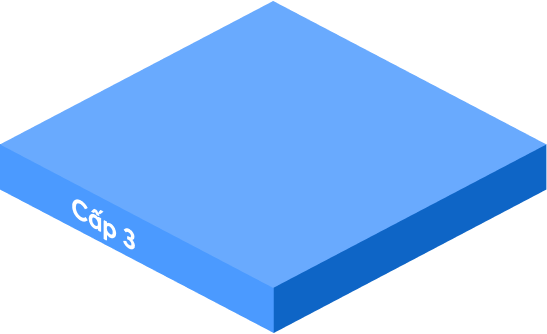
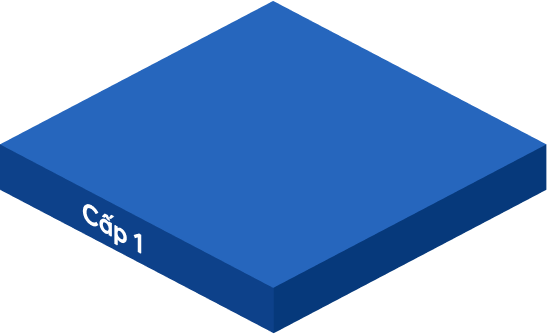
Statements from company leaders, GMP documentation system policies, color master records, ISO quality manuals and papers pertaining to quality management, manufacturing operations, operational purposes, mission vision, and strategic direction of the firm are all included.

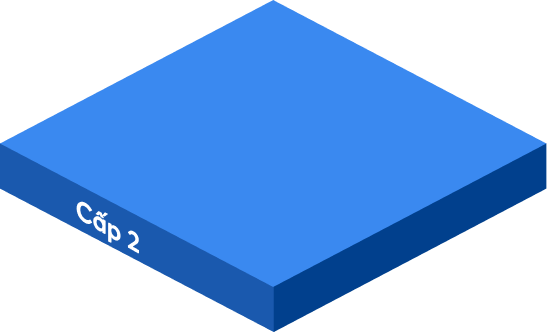
SOPs (standard operating procedures), rules, and standards for raw materials, packaging, semi-finished products, and final products based on standard reference documents are included.

Includes a system of records kept on forms throughout the facility's production, testing, and preservation processes.
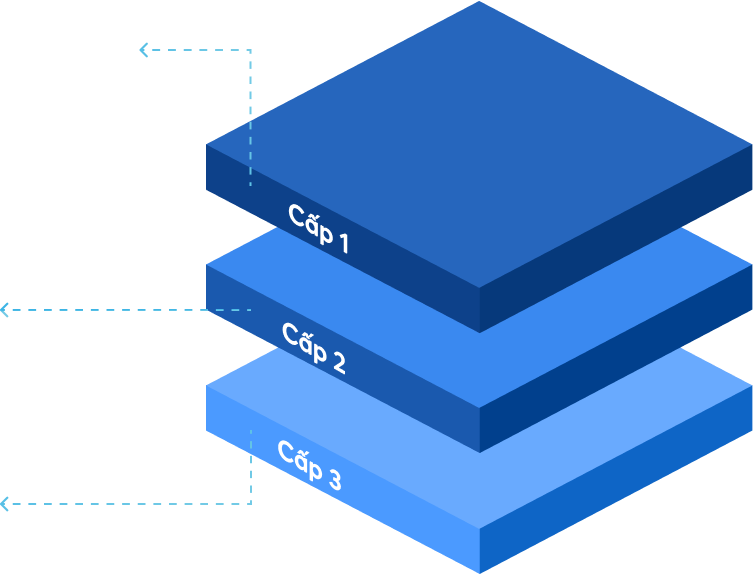
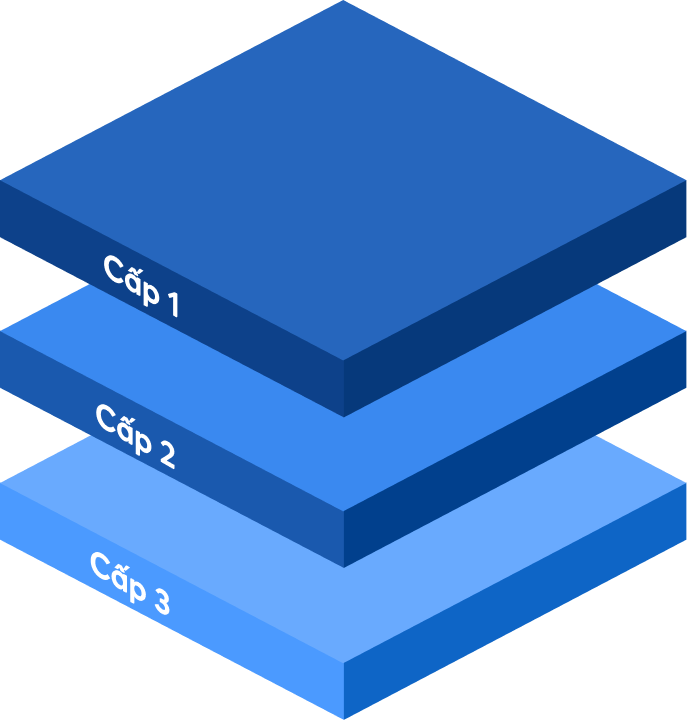
Statements from company leaders, GMP documentation system policies, color master records, ISO quality manuals and papers pertaining to quality management, manufacturing operations, operational purposes, mission vision, and strategic direction of the firm are all included.
SOPs (standard operating procedures), rules, and standards for raw materials, packaging, semi-finished products, and final products based on standard reference documents are included.
Includes a system of records kept on forms throughout the facility's production, testing, and preservation processes.